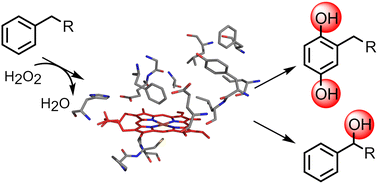
Over the last few years, unspecific peroxygenases (UPOs) have gained increasing attention from both chemists and biotechnologists, because they enable oxidation of various organic compounds at the expense of hydrogen peroxide and do not require expensive redox cofactors. While UPOs accept diverse substrates and enable a broad range of oxygenation reactions, aromatic hydroxylation reactions catalysed by these enzymes have been rarely described.
Fabian Schmitz has identified and characterized an unspecific peroxygenases from Aspergillus brasiliensis (AbrUPO) that is able to catalyse aromatic hydroxylation of substituted benzenes. The preference of AbrUPO for aromatic or benzylic hydroxylation was found to depend on the number, chemical properties and length of existing ring substituents. The publication recently appeared in Reaction Chemistry & Engineering. Below you can read the abstract. The link to the whole publication can be found in the reference at the bottom.
"Selective aromatic hydroxylation of substituted benzenes provides access to versatile phenolic synthons. Unspecific peroxygenases (UPOs) have been recognised as promising biocatalysts for synthetic chemistry. While UPOs accept diverse substrates and enable a broad range of oxygenation reactions, aromatic hydroxylation reactions catalysed by these enzymes have been rarely described. Here, we report on a UPO from Aspergillus brasiliensis (AbrUPO) heterologously expressed in Pichia pastoris at a concentration of 742 mg per litre that is able to catalyse aromatic hydroxylation of substituted benzenes. The preference of AbrUPO for aromatic or benzylic hydroxylation was found to depend on the number, chemical properties and length of existing ring substituents. While oxidation of ethylbenzene gave ring- and side-chain hydroxylation products at a 1 : 1 ratio, increasing the chain-length of the alkyl substituent enhanced the preference for benzylic hydroxylation. With the para-disubstituted p-cymene as a substrate, the chemoselectivity of AbrUPO strongly shifted towards aromatic hydroxylation. All tested substituted phenols resulted in exclusive aromatic hydroxylation. The observed formation of low quantities of quinones was attributed to the inherent peroxidase activity, while further oxidation of benzylic alcohols to ketones was suggested to occur due to both peroxidase and peroxygenase activity of AbrUPO. ‘Overoxidation’ due to peroxidase activity could be completely avoided by adding ascorbic acid and shortening reaction time."
Schmitz F, Koschorreck K, Hollmann F, Urlacher VB, 2023, Aromatic hydroxylation of substituted benzenes by an unspecific peroxygenase from Aspergillus brasiliensis, Reaction Chemistry & Engineering, https://doi.org/10.1039/D3RE00209H
© 2023 The Authors. This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. You can use material from this article in other publications without requesting further permissions from the RSC, provided that the correct acknowledgement is given.