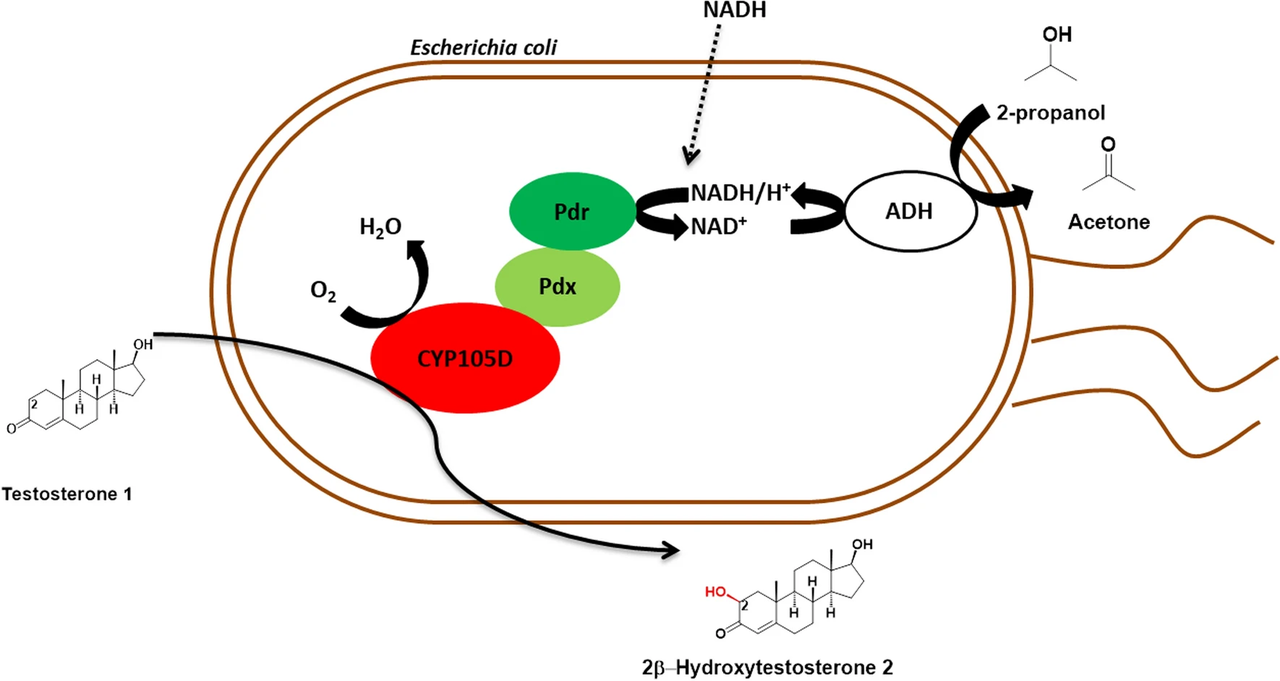
A team of scientists from our institute compared wet resting cells and lyophilized cells of recombinant E. coli regarding P450-catalyzed oxidation and found out that lyophilized cells are well-appropriate as P450-biocatalysts. The team could demonstrate that (i) handling procedure has a strong effect on the catalytic performance of recombinant P450-containing resting cells, (ii) lyophilized recombinant E. coli cells can be used for P450-mediated biocatalysis, when (iii) metabolism-independent regeneration of NAD(P)H is ensured. The publication recently appeared in AMB Express. Below you can read the abstract. The link to the whole publication can be found in the reference at the bottom.
"Cytochromes P450 catalyze oxidation of chemically diverse compounds and thus offer great potential for biocatalysis. Due to the complexity of these enzymes, their dependency of nicotinamide cofactors and redox partner proteins, recombinant microbial whole cells appear most appropriate for effective P450-mediated biocatalysis. However, some drawbacks exist that require individual solutions also when P450 whole-cell catalysts are used. Herein, we compared wet resting cells and lyophilized cells of recombinant E. coli regarding P450-catalyzed oxidation and found out that lyophilized cells are well-appropriate as P450-biocatalysts. E. coli harboring CYP105D from Streptomyces platensis DSM 40041 was used as model enzyme and testosterone as model substrate. Conversion was first enhanced by optimized handling of resting cells. Co-expression of the alcohol dehydrogenase from Rhodococcus erythropolis for cofactor regeneration did not affect P450 activity of wet resting cells (46% conversion) but was crucial to obtain sufficient P450 activity with lyophilized cells reaching a conversion of 72% under the same conditions. The use of recombinant lyophilized E. coli cells for P450 mediated oxidations is a promising starting point towards broader application of these enzymes."
Hilberath T, Raffaele A, Windeln LM, Urlacher VB, 2021, Evaluation of P450 monooxygenase activity in lyophilized recombinant E. coli cells compared to resting cells, AMB Express., 11(1):162, https://doi.org/10.1186/s13568-021-01319-0