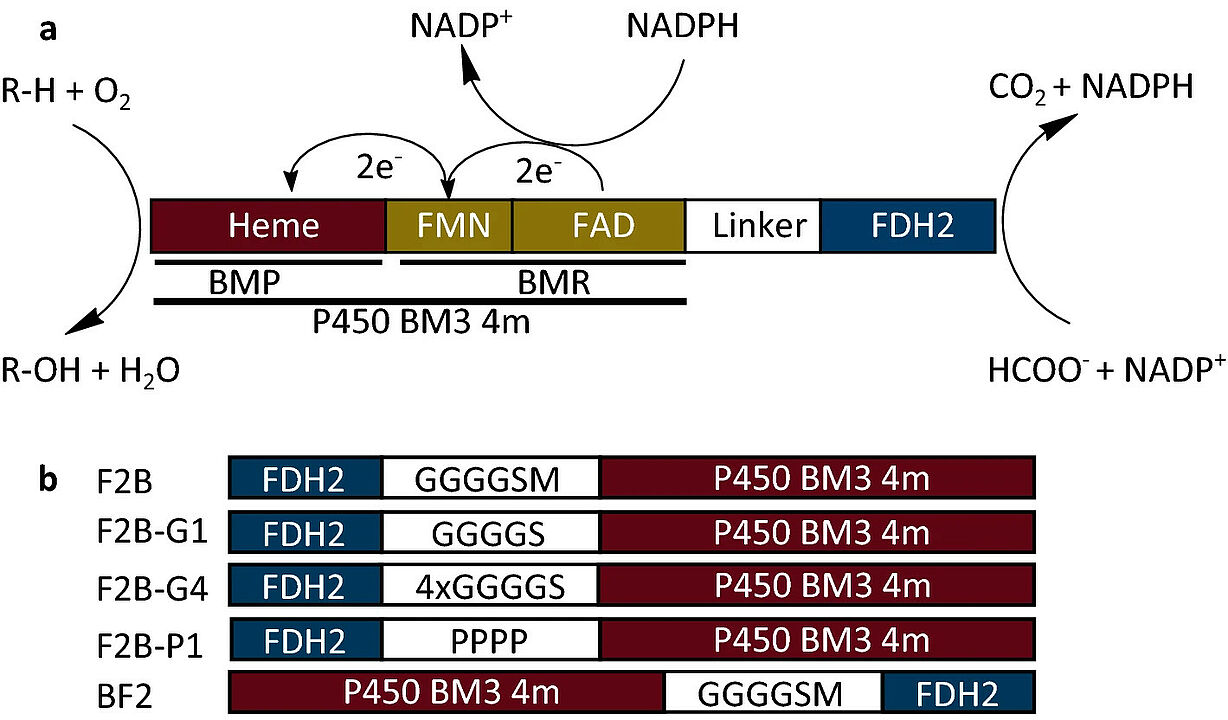
Arsenij Kokorin successfully fused the genes coding for cytochrome P450 BM3 from B. megaterium and formate dehydrogenase from Pseudomonas sp. to enable both substrate oxidation catalyzed by P450 BM3 and continuous cofactor regeneration by formate dehydrogenase within one construct. Most noticeably, an activity increase of up to threefold was observed for the fusion constructs with various substrates which were partly attributed to the increased diflavin reductase activity of the P450 BM3. The results recently appeared in Scientific Reports. Below you can read the abstract. The link to the whole publication can be found in the reference at the bottom.
"Fusion of multiple enzymes to multifunctional constructs has been recognized as a viable strategy to improve enzymatic properties at various levels such as stability, activity and handling. In this study, the genes coding for cytochrome P450 BM3 from B. megaterium and formate dehydrogenase from Pseudomonas sp. were fused to enable both substrate oxidation catalyzed by P450 BM3 and continuous cofactor regeneration by formate dehydrogenase within one construct. The order of the genes in the fusion as well as the linkers that bridge the enzymes were varied. The resulting constructs were compared to individual enzymes regarding substrate conversion, stability and kinetic parameters to examine whether fusion led to any substantial improvements of enzymatic properties. Most noticeably, an activity increase of up to threefold was observed for the fusion constructs with various substrates which were partly attributed to the increased diflavin reductase activity of the P450 BM3. We suggest that P450 BM3 undergoes conformational changes upon fusion which resulted in altered properties, however, no NADPH channeling was detected for the fusion constructs."
Kokorin A, Parshin PD, Bakkes PJ, Pometun AA, Tishkov VI, Urlacher VB, 2021, Genetic fusion of P450 BM3 and formate dehydrogenase towards self-sufficient biocatalysts with enhanced activity, Scientific Reports, 11(1):21706, https://doi.org/10.1038/s41598-021-00957-5