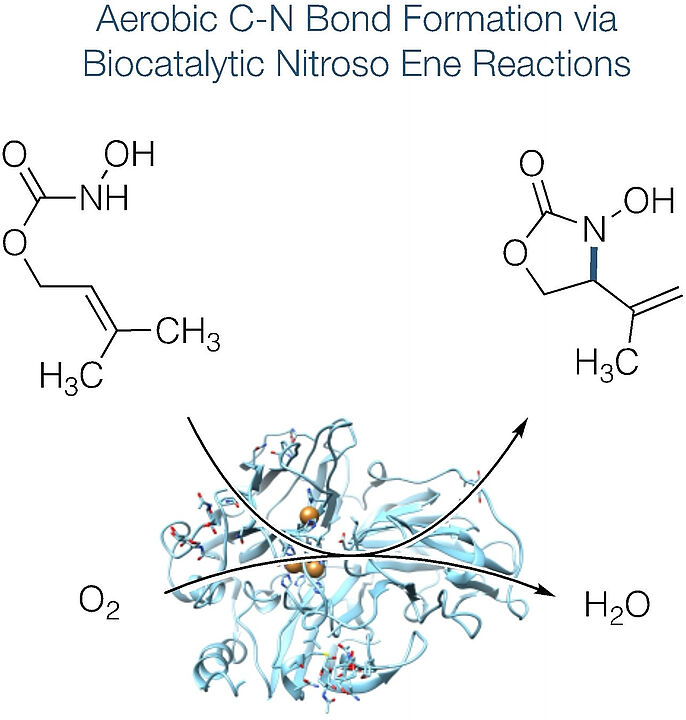
Scientists from our group together with collegues from Aalto University and the University of Helsinki investigated on different pathways of two biocatalytic systems (laccases and an oxidase/peroxidase couple) to provide more insight into the unprecedented promiscuity of classical oxidoreductases as catalysts for nitroso-based transformations. The publication recently appeared in Angewandte Chemie Internationale Edition. Below you can read the abstract. The link to the whole publication can be found in the reference at the bottom.
"The biocatalytic oxidation of acylated hydroxylamines enables the direct and selective introduction of nitrogen functionalities by activation of allylic C-H bonds. Utilizing either laccases or an oxidase/peroxidase couple for the formal dehydrogenation of N-hydroxycarbamates and hydroxamic acids with air as the terminal oxidant, acylnitroso species are generated under particularly mild aqueous conditions. The reactive intermediates undergo C-N bond formation through an ene-type mechanism and provide high yields both in intramolecular and intermolecular enzymatic aminations. Investigations on different pathways of the two biocatalytic systems and labelling studies provide more insight into this unprecedented promiscuity of classical oxidoreductases as catalysts for nitroso-based transformations. "
Jäger C, Haase M, Koschorreck K, Urlacher VB, Deska J, 2023, Aerobic C-N Bond Formation through Enzymatic Nitroso-Ene-Type Reactions, Angew Chem Int Ed, e202213671, https://doi.org/10.1002/anie.202213671