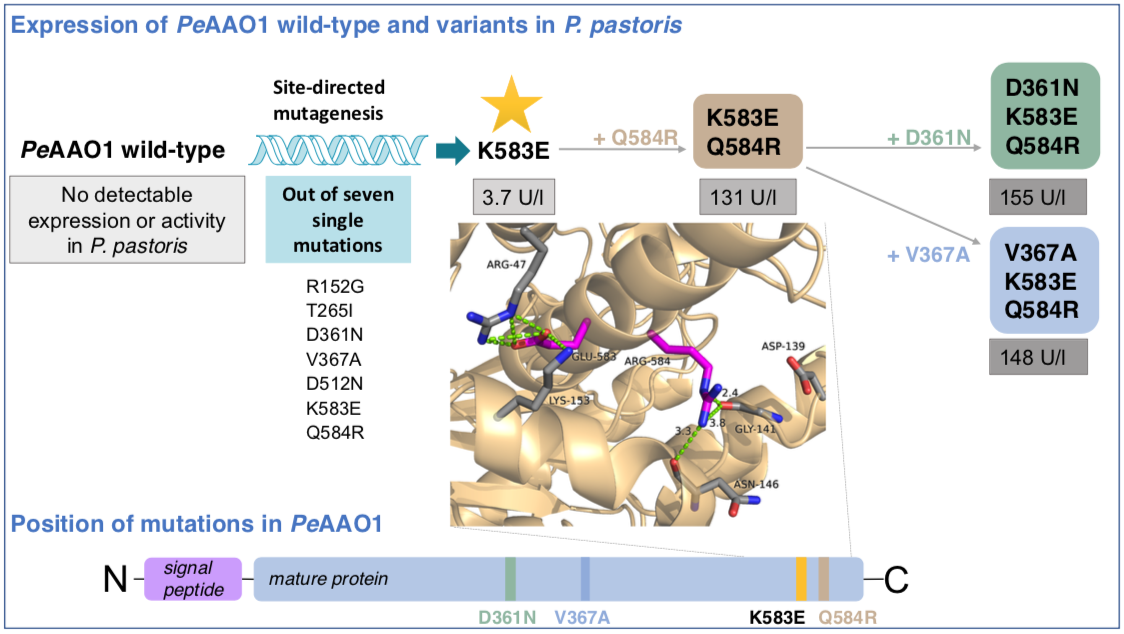
By successively introducing mutations, Nina Jankowski and Katja Koschorreck successfully expressed an aryl-alcohol oxidase from Pleurotus eryngii in Pichia pastoris. Functional expression was enhanced up to 155 U/l during the experiments. The results recently appeared in Applied Microbiology and Biotechnology.
"Fungal aryl-alcohol oxidases (AAOs) are attractive biocatalysts because they selectively oxidize a broad range of aromatic and aliphatic allylic primary alcohols while yielding hydrogen peroxide as the only by-product. However, their use is hampered by challenging and often unsuccessful heterologous expression. Production of PeAAO1 from Pleurotus eryngii ATCC 90787 in Pichia pastoris failed, while PeAAO2 from P. eryngii P34 with an amino acid identity of 99% was expressed at high yields. By successively introducing mutations in PeAAO1 to mimic the sequence of PeAAO2, the double mutant PeAAO1 ER with mutations K583E and Q584R was constructed, that was successfully expressed in P. pastoris. Functional expression was enhanced up to 155 U/l via further replacements D361N (variant NER) or V367A (variant AER). Fed-batch cultivation of recombinant P. pastoris yielded up to 116 mg/l of active variants. Glycosylated PeAAO1 variants demonstrated high stability and catalytic efficiencies similar to PeAAO2. Interestingly, P. pastoris expressing PeAAO1 variant ER contained roughly 13 gene copies but showed similar volumetric activity as NER and AER with one to two gene copies and four times lower mRNA levels. Additional H-bonds and salt bridges introduced by mutations K583E and Q584R might facilitate heterologous expression by enhanced protein folding."
Jankowski N, Urlacher VB, Koschorreck K, 2021, Two adjacent C-terminal mutations enable expression of aryl-alcohol oxidase from Pleurotus eryngii in Pichia pastoris, Applied Microbiology and Biotechnology, https://doi.org/10.1007/s00253-021-11585-4