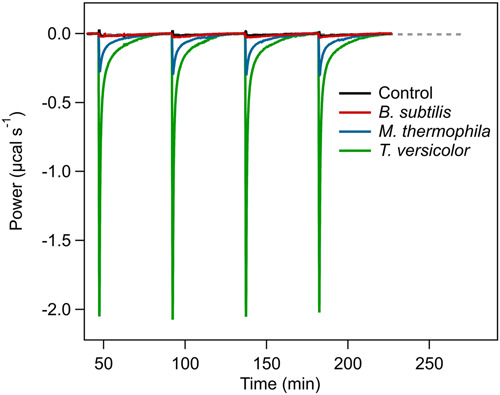
A team of scientists successfully performed isothermal titration calorimetric assessment of lignin conversion by laccases. As Lignin valorization may offer a sustainable approach to achieve a chemical industry that is not completely dependent on fossil resources for the production of aromatics the group investigated on the differences in the extent and rate of conversion for the different enzymes. Prof. Urlacher wrote together with Shams T. A. Islam, Jie Zhang and Peter-Leon Hagedoorn the manuscript for this publication which recently appeared in Biotechnology and Bioengineering. Below you can read the abstract. The link to the whole publication can be found in the reference at the bottom.
"Lignin valorization may offer a sustainable approach to achieve a chemical industry that is not completely dependent on fossil resources for the production of aromatics. However, lignin is a recalcitrant, heterogeneous, and complex polymeric compound for which only very few catalysts can act in a predictable and reproducible manner. Laccase is one of those catalysts and has often been referred to as an ideal "green" catalyst, as it is able to oxidize various linkages within lignin to release aromatic products, with the use of molecular oxygen and formation of water as the only side product. The extent and rate of laccase-catalyzed lignin conversion were measured using the label-free analytical technique isothermal titration calorimetry (ITC). IITC provides the molar enthalpy of the reaction, which reflects the extent of conversion and the time-dependent power trace, which reflects the rate of the reaction. Calorimetric assessment of the lignin conversion brought about by various fungal and bacterial laccases in the absence of mediators showed marked differences in the extent and rate of conversion for the different enzymes. Kraft lignin conversion by Trametes versicolor laccase followed Michaelis-Menten kinetics and was characterized by the following thermodynamic and kinetic parameters ΔHITC = -(2.06 ± 0.06)·103 kJ mol-1 , KM = 6.6 ± 1.2 μM and Vmax = 0.30 ± 0.02 U/mg at 25°C and pH 6.5. We envision calorimetric techniques as important tools for the development of enzymatic lignin valorization strategies."
A Islam ST, Zhang J, Tonin F, Hinderks R, Deurloo YN, Urlacher VB, Hagedoorn PL, 2022, Isothermal titration calorimetric assessment of lignin conversion by laccases, Biotechnology and Bioengineering, 119(2):493–503, https://doi.org/10.1002/bit.27991